PARIS, Oct. 29, 2015 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
|
Q3 2015 |
Change (reported) |
Change | |
|
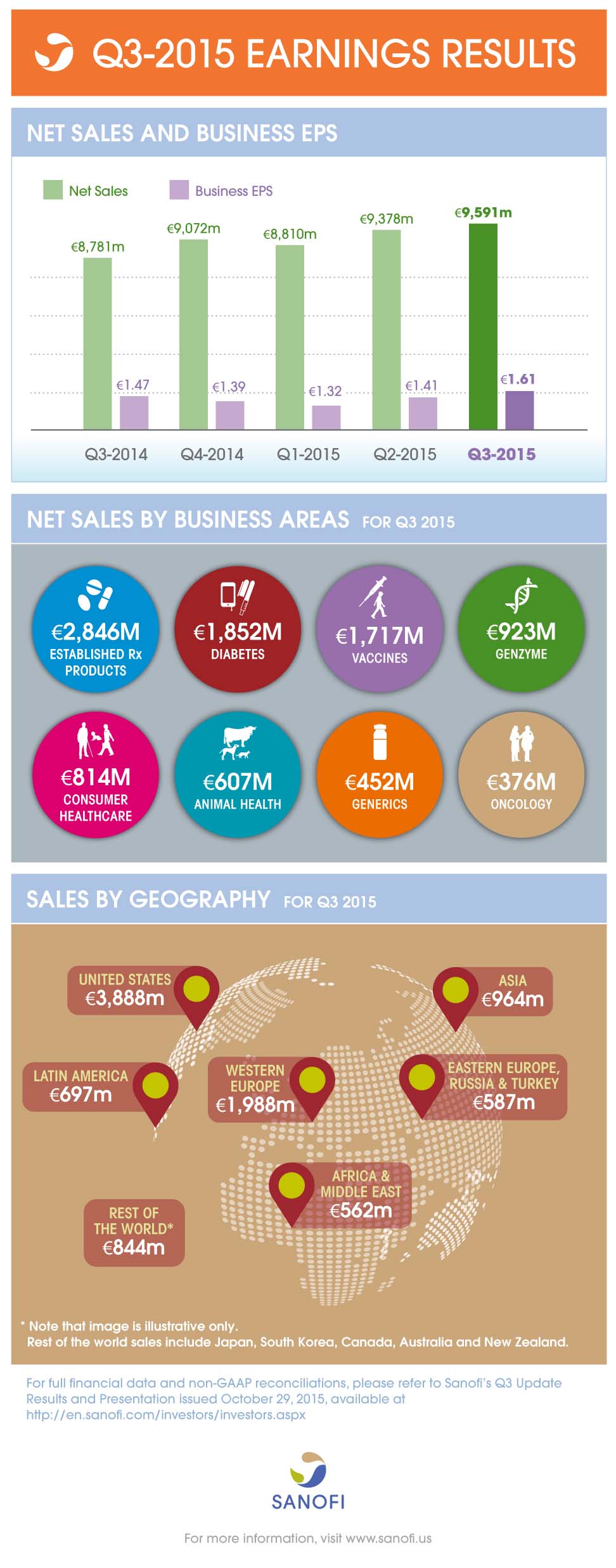
Net sales |
€9,591m |
+9.2% |
+3.4% |
|
Business net income(1) |
€2,096m |
+8.3% |
+5.0% |
|
Business EPS(2) |
€1.61 |
+9.5% |
+6.1% |
In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income(1) is a non-GAAP financial measure. (2)(EPS) Earnings Per Share
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7661751-sanofi-results-q3-2015/

Sanofi Chief Executive Officer, Dr. Olivier Brandicourt, commented:
"The Group showed growth on both top and bottom line in the third quarter driven by strong performance of Genzyme, Vaccines and Emerging Markets. At the same time, we continue to make significant investments to strengthen Sanofi for the future. With the growing adoption of new products such as Aubagio®, NexGard®, Lemtrada®, and Toujeo® and the recent launch of Praluent®, we have achieved important milestones in our mission to bring innovative medicines to patients. Despite headwinds in our diabetes business, we are confident in Sanofi's long-term prospects and we look forward to sharing our roadmap for the Group on November 6, 2015."
Broad-based sales growth despite diabetes sales erosion in the U.S.
- Group sales increased 3.4% (up 9.2% on a reported basis) to €9,591 million
- Diabetes sales decreased 6.6%, as a result of lower U.S. sales of Lantus®
- Genzyme sales (up 32.7%) showed strong momentum driven by Multiple Sclerosis products
- Vaccines sales were up 5.5% mainly driven by Emerging Markets
- Animal Health delivered another strong performance (up 9.3%) driven by NexGard®
- Emerging Markets sales increased by 11.4%
Steady financial performance taking into account higher investments in new products
- Operating expenses were €3,816m, up 7.5% at CER
- Business net income grew 5.0% at CER (up 8.3% on a reported basis) to €2,096 million
- Business EPS increased 6.1% at CER to €1.61 and grew 9.5% on a reported basis
- Subsequent event - The financial impact of a voluntary recall announced yesterday for Auvi-Q® and Allerject® in the U.S. and Canada is under evaluation and will be accounted for in Q4 2015. An initial estimate is a negative impact of approximately €100m on Business Net Income
Significant progress in advancing innovative products
- Praluent® launched in the U.S. in July and first EU launches underway
- New Drug Application for lixisenatide accepted for review by the FDA
- Primary endpoint met in second Phase III study for LixiLan in Type 2 diabetes
2015-2018 diabetes outlook
- Accounting for recent market trends, Sanofi now projects global diabetes sales over the period of 2015-2018 to decline at an average annualized rate of between 4% and 8% at CER. Sanofi will mitigate the impact of this revised sales expectation on its business operating income by 2018 and will present the mid-term strategic and financial outlook for the Group on November 6, 2015
R&D Update
Regulatory update
Regulatory updates since the publication of the second quarter results on July 30, 2015 include the following:
- In October, VaxiGrip® QIV (Quadrivalent inactivated influenza vaccine) for children three years old or above was submitted to European authorities.
- In September, the U.S. Food and Drug Administration (FDA) accepted for filing the New Drug Application (NDA) for lixisenatide, an investigational once-daily prandial GLP-1 receptor agonist for the treatment of adults with type 2 diabetes.
- In September, the European Commission (EC) granted marketing authorization for Praluent® (alirocumab, collaboration with Regeneron) for the treatment in certain adult patients of hypercholesterolemia characterized by high level of low-density lipoprotein (LDL) cholesterol. This approval follows the FDA approval received on July 24th. In August, Praluent® was also submitted to Japanese health authorities.
At the end of October 2015, the R&D pipeline contained 41 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 13 are in Phase III or have been submitted to the regulatory authorities for approval.
Collaboration
- In August, Sanofi and Google Life Sciences announced that the companies are collaborating to improve care and outcomes for people with type 1 and type 2 diabetes. The collaboration will pair Sanofi's leadership in diabetes treatments and devices with Google's expertise in analytics, miniaturized electronics and low power chip design. The companies will explore how to improve diabetes care by developing new tools that bring together many of the previously siloed pieces of diabetes management and enable new kinds of interventions. This includes health indicators such as blood glucose and hemoglobin A1c levels, patient-reported information, medication regimens and sensor devices.
- In August, Sanofi also entered a research collaboration and license agreement with Evotec and Apeiron Biologics to discover and develop first-in-class small molecule-based immuno-oncology therapies to treat solid and hematological cancers by enhancing the anti-tumor activity of the human immune system.
- In August, Sanofi entered a strategic research collaboration with Evotec to develop beta cell-modulating diabetes treatments, which may reduce or eliminate the need for insulin injections.
2015 Guidance
- Sanofi reaffirms that it expects 2015 Business EPS(2) to be stable to slightly growing versus 2014 at constant average exchange rates, barring unforeseen major adverse events
- In addition, the positive currency impact on 2015 full-year business EPS is estimated to be between 6% and 8%, under the assumption that exchange rates remain stable in fourth quarter at the average rates of September 2015
To access the full press release of the Q3 2015 results, please click here.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group's ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2014. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.


SOURCE Sanofi
