PARIS, Oct. 15, 2019 /PRNewswire/ -- Sanofi today celebrates the inauguration of its new digital manufacturing facility in Framingham, Massachusetts, marking one of the world's first digital facilities using intensified, continuous biologics production technology.
Experience the interactive Multichannel News Release here:
https://www.multivu.com/players/English/8627651-sanofi-new-digital-manufacturing-facility/
The new facility features leading-edge technology that connects the production process with research and development, paving the way for improved commercialization of important new medicines for patients.
This facility accelerates the recent transformation of Sanofi's Industrial Affairs organization to focus on biologics-based therapies, in line with the transformation of the company's R&D pipeline. The ramping up of bio-pharmaceutical production capacities is a key pillar to achieving Sanofi's ambition to establish the gold standard in the bio-pharmaceutical industry.
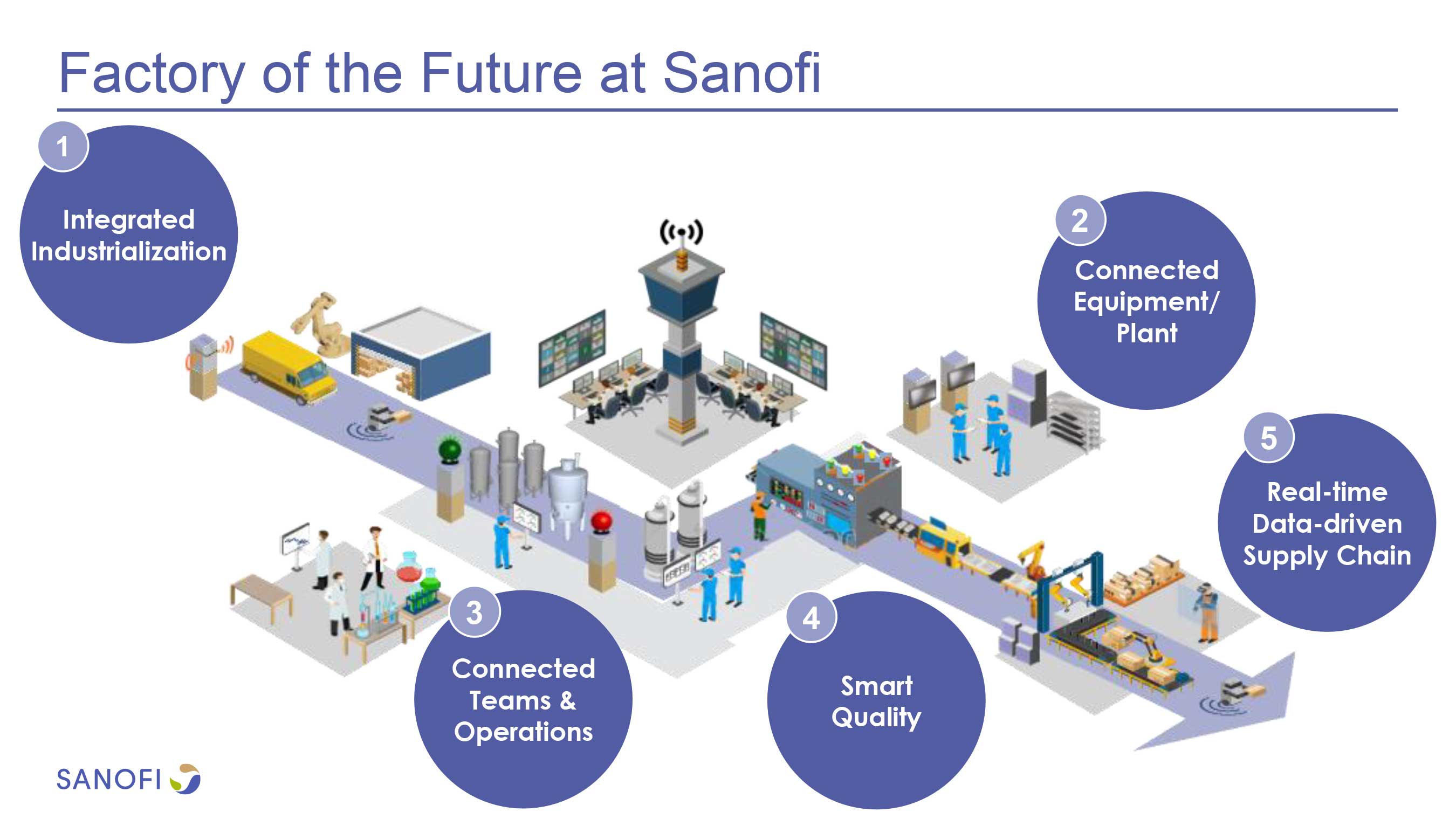
Sanofi at the leading edge of biologics manufacturing
The facility's advanced paperless and data-driven manufacturing technologies is expected to enable Sanofi to achieve higher levels of productivity, agility, and flexibility, reducing the time it takes for products to move from the development labs to the manufacturing plant, and – most importantly – meeting the needs of patients around the world.
"We have been investing for some years to prepare for Sanofi's future. Our Framingham facility leads the way in delivering the next generation of biologics manufacturing, leveraging intensified, continuous processing in a fully integrated digitally powered facility," said Philippe Luscan, Executive Vice President, Global Industrial Affairs at Sanofi. "This opening demonstrates we are at the leading edge of innovation and manufacturing excellence, helping us to shape the future of both our company and the industry."
The digital transformation of Sanofi's manufacturing network is a key element of the company's goal to leverage better use of data to optimize the company's manufacturing processes, increasing efficiencies, improving the agility needed to respond to fast changing patient needs, and speeding up the commercialization of new medicines emerging from the R&D pipeline.
Framingham is the latest biologics manufacturing facility amongst a number of pilots, which are being accelerated across the Sanofi network. The innovations established at this facility are being rapidly deployed and standardized across the company. Framingham is the first "digitally born" facility while similar digital transformations are introduced in other legacy plants. Beyond Framingham, Sanofi intends to move forward with digital transformation initiatives in Toronto (Canada), Suzano (Brazil), Waterford (Ireland), Sisteron (France), and Geel (Belgium).
Newest part of Sanofi's integrated biologics hub in Framingham
The Framingham digital bio-manufacturing facility is part of Sanofi's integrated, cross-functional biologics hub in Framingham, Massachusetts. A critical global hub for more than 30 years, the campus offers co-location of all the infrastructure and activities required to speed the delivery of innovative new therapies to patients, from early stage research and process development through clinical and commercial manufacturing, including the key enabling functions of quality control and compliance, regulatory, engineering, supply chain, and learning and development.
The co-location of these critical functions allows for seamless, end-to-end product and process design and manufacturing and provides leading-edge development opportunities for our employees' competencies evolution.
About Sanofi
Sanofi is dedicated to supporting people through their health challenges. We are a global biopharmaceutical company focused on human health. We prevent illness with vaccines, provide innovative treatments to fight pain and ease suffering. We stand by the few who suffer from rare diseases and the millions with long-term chronic conditions.
With more than 100,000 people in 100 countries, Sanofi is transforming scientific innovation into healthcare solutions around the globe.
Sanofi, Empowering Life
Media Relations Contact | Investor Relations Contact |
Nicolas Kressmann | George Grofik |
Tel.: +1 (732) 532-5318 | Tel.: +33 (0)1 53 77 45 45 |
Sanofi Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi's ability to benefit from external growth opportunities, to complete related transactions and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic conditions, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2018. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Source: Sanofi (EURONEXT: SAN) (NASDAQ: SNY)





SOURCE Sanofi



