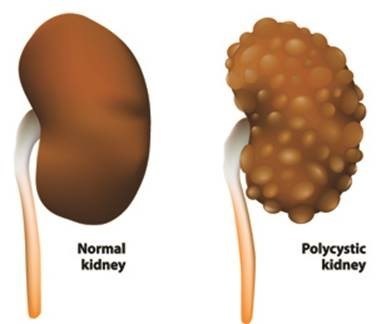
BRIDGEWATER, N.J., Oct. 26, 2018 /PRNewswire/ -- Autosomal dominant polycystic kidney disease (ADPKD) is a devastating rare genetic kidney condition that leads to the growth of numerous cysts in the kidneys. Affecting an estimated 120,000 people in the U.S. and 170,000 in the European Union, ADPKD becomes so severe for approximately half of those patients that they face either a lifetime of dialysis or a kidney transplant.

Sanofi is beginning a pivotal clinical trial to study the safety, efficacy, and tolerability of an investigational oral agent called venglustat for certain patients with ADPKD. The international trial is enrolling patients who are at risk of rapidly progressive ADPKD.
"The initiation of this clinical trial is another reflection of Sanofi's commitment to research, advanced scientific discovery, and true innovation," said Gianluca Pirozzi, Head of Development for Rare Diseases and Head of Translational Gene Therapy, Sanofi Genzyme, the specialty care global business unit of Sanofi. "Our understanding thus far of both the cause and progression of ADPKD and the mechanism of action of venglustat present us with a path forward in this research effort."
Genetic mutation leads to devastating condition
ADPKD is caused by a mutation in the PKD1 or PKD2 gene that leads to a build-up of complex substances called glycosphingolipids in the kidneys. Glycosphingolipid accumulation is thought to be an important driver of cyst growth1,2. Relentless cyst growth can cause chronic pain and lead to reduced kidney function and kidney failure in ADPKD patients. The symptoms of ADPKD usually start to appear between the ages of 30 and 40, but they can begin as early as childhood for some patients.3
"The PKD Foundation welcomes research efforts that have the potential to bring new therapies to patients living with this condition," said David Baron, Ph.D., Chief Scientific Officer of the PKD Foundation. "We appreciate that Sanofi has engaged with the ADPKD patient community throughout the early stages of clinical development for venglustat and look forward to continuing to work with Sanofi."
About Venglustat
Venglustat is an investigational oral therapy designed to inhibit the abnormal accumulation of a substance in the body called glucosylceramide (GL-1), which plays a role in production of glycosphingolipids. In genetic mouse models of ADPKD, inhibition of glycosphingolipid production has been shown to reduce kidney cyst growth.4 The clinical significance of this is under investigation.
"Venglustat represents a potential opportunity for Sanofi Genzyme to expand its core legacy of expertise in lysosomal storage disorders and make an impact on patients living with other rare and challenging diseases," said Sébastien Martel, Global Head of Rare Diseases, Sanofi Genzyme. "Our progress related to evaluating venglustat in ADPKD once again highlights our company-wide commitment to continually build on our experience and focus our research efforts on unmet needs for patients around the world."
Venglustat has received Orphan Drug designation in the U.S. for the treatment of ADPKD. The ADPKD clinical trial will be conducted at sites in the U.S., Canada, China and Japan as well as several EU countries.
For more information on this trial, please visit https://www.clinicaltrials.gov or https://www.clinicaltrialsregister.eu. U.S. patients interested in learning more, may also visit https://adpkdtrial.org.
Learn more about ADPKD:
PKD Foundation: https://pkdcure.org/
American Kidney Fund: http://www.kidneyfund.org/
National Kidney Foundation: https://www.kidney.org/
National Institutes of Health: https://www.niddk.nih.gov/health-information/kidney-disease/polycystic-kidney-disease/autosomal-dominant-pkd
1 Chatterjee S, Shi WY, Wilson P, Mazumdar A. Role of lactosylceramide and MAP kinase in the proliferation of proximal tubular cells in human polycystic kidney disease. J Lipid Res. 1996;37(6):1334-44
2 Deshmukh GD, Radin NS, Gattone VH, 2nd, Shayman JA. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J Lipid Res. 1994;35(9):1611-8.
4 Natoli TA, Smith LA, Rogers KA, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010 Jul;16(7):788-92.
SOURCE Sanofi