PARIS, May 14, 2018 /PRNewswire/ -- Clinical trials are the crucial link between a discovery in the research lab and a new treatment reaching patients. Regulatory authorities, like the Food & Drug Administration in the U.S., require these studies to demonstrate a therapy's safety and effectiveness before it goes to market.
They are also taking more time than ever to complete across the industry, making patients in need wait longer for a promising new therapy. The process, which can take years, contributes to an increase in the cost of bringing medicines to market by about 10 percent annually.1
In an effort to meet patient needs and reduce costs, pharmaceutical companies are finding ways to become more collaborative with medical center partners. Medical centers are on the front lines of clinical studies. They are responsible for identifying appropriate patients, executing against the study protocols, and collecting all the complex data from the study.
The more closely the two collaborate, the more likely that studies will be more efficient in moving from design to regulatory submission.
"I've seen a shift over the last five years, where companies are increasingly seeing the value of the voice of the clinical trial site," said Karri Venn, President, Research at LMC Manna Research, which has worked closely with Sanofi on its clinical studies. "Across our trials, we see the full gamut of examples of what works well and can share that intelligence with industry."
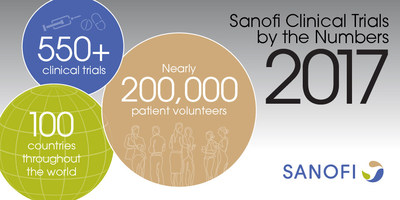
For a global company like Sanofi, streamlining clinical trials is a big undertaking. The company conducted more than 550 clinical trials last year, involving nearly 200,000 patients; work with medical centers and other clinical research sites keeps a team of 2,100 Sanofi employees busy across the globe.
Acting on insights from LMC Manna Research and others, Sanofi is undergoing a key initiative to make it easier for clinical sites to conduct its trials. Called "Sanofi Makes Investigators Lives Easier," or SMILE, its goal is to reduce the burden placed on research site staff so they can focus more time with study participants.
"Our work is ultimately for patients, and no one interacts more closely with them than clinical sites," said Kelly Simcox, head of the Americas Clinical Study Unit at Sanofi. "We want to effectively address site needs, make it easier for their staff, and collaborate to make potential treatment advances available sooner for patients."
Clinical Trials: The Need for Public Awareness
One important aspect of collaboration is creating greater public awareness of clinical trials, in order to broaden the number of potential patients available for a trial and to encourage more people to volunteer to participate.
"Many people don't always consider whether there are clinical studies underway for their particular disease," said Simcox. "In trials, patient recruitment is one of the most important factors affecting how long trials run. The more we can raise awareness, the greater the chances for success."
Traditional approaches to awareness involve ads on radio or community newspapers. To amplify the reach, Sanofi is helping sites recruit study participants across social media. Interested individuals can complete an online questionnaire to determine if they meet the study's criteria.
Another aspect of the SMILE initiative is developing new approaches that make it easier to recruit study participants once they've been qualified. That means overcoming concerns such as how much time participants must commit. To address these logistical challenges, Sanofi regularly conducts patient focus groups to understand what makes a study burdensome, including the number of office visits or types of procedures required in a trial.
To support SMILE, the company has built a Global Investigator Network, which helps to identify ways to improve study practices, such as accelerating recruitment. This network includes a total of 95 medical sites from 21 countries. These centers are eager to work with Sanofi to make a difference for their staff and ultimately, their patients.
Sanofi received recognition for all its efforts late last month – the company earned second place in the Association for Clinical Research Professionals (ACRP) and The Avoca Group 2018 Sponsor Quality award category. Clinical research site members of ACRP nominated Sanofi for the company's commitment to help them effectively execute clinical studies.
By advocating for clinical sites and their patients, Sanofi aims to accelerate the development of potential new therapies for people who need them.
"If we can cut clinical trial timelines and improve the experience for clinical research sites and patients, everyone wins," said Venn.
References:
1 Tufts Center for the Study of Drug Development
Sanofi Media Relations:
Anna Robinson
anna.robinson@sanofi.com
908-229-6871

SOURCE Sanofi